Pilot Study
- Home
- Project
- Pilot Study
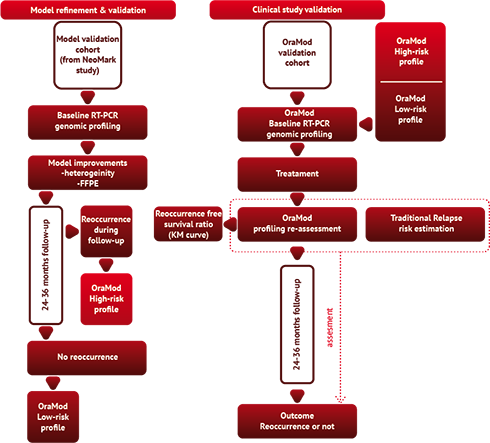
The OraMod concept will be tested and validated through a clinical observational study in a relevant number of Oral Squamous Cell Carcinoma cases treated with conventional surgery ± radiation and followed up for at least 24 months. The assessment will be verified in a clinical study carried out at the clinical centers of Parma, Dusseldorf, Amsterdam and Tampere.
The study will take advantage of two distinct cohorts: a retrospective cohort used to train and internally validate the model and an independent cohort ("study cohort") of patients specifically selected for OraMod study. The model validation cohort will be composed of 150 cases with complete data from the NeoMark study and from retrospective cases from other participating centers. The OraMod "study cohort" will include approximately 120-140 cases, selected from the over 560 cases available at VUmc, Parma University Hospital, Dusseldorf Heinrich-Heine University Clinic and the Finnish Hospital of Tampere, of whom at least three years of clinical follow-up is available. Both cohorts will include patients diagnosed with oral squamous cell carcinoma (OSCC) followed in the OraMod clinics according to state-of-art therapeutic approach as from international guidelines. Based on previous experiences in participating centers and on literature, we expect a reoccurrence rate in the order of 30%.
Three phases are considered:
(i) Model refinement and validation from an initial set of 150 retrospective cases. Data analysis and the risk prediction model will be validated.
(ii) OraMod study cohort enrolment and baseline evaluation: patient's data will be collected at the time of diagnosis and tumor specimens analyzed in each center and recorded using OraMod platform. The risk prediction will be assessed both by traditional methodology and by using OraMod predictive modeling and simulation. Follow-up data will also be recorded using OraMod platform.
(iii) Prediction accuracy assessment: at the end of the study, the status of patient (reoccurrence/no reoccurrence) will be clinically assessed and compared to predictions.
An additional verification will be done on a sub-set of formalin-embedded tissue specimens, to verify whether the extracted RNA has sufficient quality for genomic data analysis and if it produces comparable genomic markers as from fresh frozen tissues.


